Introduction
In 2020 the European Commission published a report by its Joint Research Centre. The report is based on the EU Directive 2010/63/EU on the protection of animals used for scientific purposes. The EURL ECVAM Scientific Advisory Committee (ESAC) advised against animals being used for the development and production of antibodies. This includes antibodies used research, regulatory, diagnostic, and therapeutic applications. They have also made several recommendations to promote the use of non-animal-derived antibodies. To push forward this agenda, they refuse EU to grant fund to projects involving animal-derived antibodies. Furthermore, they also restrict the publication or manuscripts in scientific journals using these products, and requesting the phasing out of animal-derived antibodies by manufacturers and suppliers. Although this is not a law in the EU, these opinions are gaining traction within the scientific community.
A small study was performed with Pivotal Scientific Limited to determine how the industry is reacting to this recommendation. This was achieved through two online surveys to antibody suppliers and distributors worldwide to gather their opinions on the topic. Life Science companies selling research use only (RUO) products were contacted and questioned about about the EURL ECVAM recommendations. Forty antibody suppliers and forty antibody distributors based in Europe, the Americas and Asia responded anonymously to share their views
Industry study
Awareness within the industry of the EURL ECVAM report was surprisingly low. There are only 30% of global suppliers and 20% of distributors who were familiar with its recommendations. When taking into account geographical location, this rose to 45% of suppliers based in Europe. Of the suppliers who responded to the survey, just under 60% already sell non-animal-derived antibodies. When asked if non-animal-derived antibodies are not only equivalent to animal-derived antibodies, but can offer significant scientific advantages and economic benefits, 60 % agreed. However, when asked if non-animal derived antibodies are ready to replace animal-derived antibodies, responses were much more cautious, 27.5% said yes, but a higher percentage of suppliers were unsure or disagreed. Similar responses were also obtained from the distributors, at 65% and 32.5% respectively. Interestingly, 10% of distributors had noticed an increase in demand and 22.5% an increase in supply for non-animal-derived antibodies over the past year.
Whilst working within the industry, PSL has observed that increasingly companies are focussing their portfolios on recombinant antibodies, with a number planning to remove non-recombinant antibodies entirely. Although recombinant antibodies are not always animal-free, synthetic or naive gene libraries allow antibody production without the need for animal immunization. Analysis of the survey results suggests that although there is a great deal of positivity towards the idea of non-animal derived antibodies, companies are far less receptive to the recommendation to replace current commercial antibodies with non-animal derived alternatives. A number of respondents raised concerns regarding the price and reliability of these antibodies.
Study analysis
Should the European Commission wish to see the removal of animals from the antibody generation and manufacturing process, a more proactive approach is required. Education of suppliers and customers to the advantages of non-animal derived antibodies may help generate awareness and customer demand. Without these, it is highly unlikely that the manufacture of animal-derived monoclonal and polyclonal antibodies will cease in the near future.
The Antibody Manufacturing Process
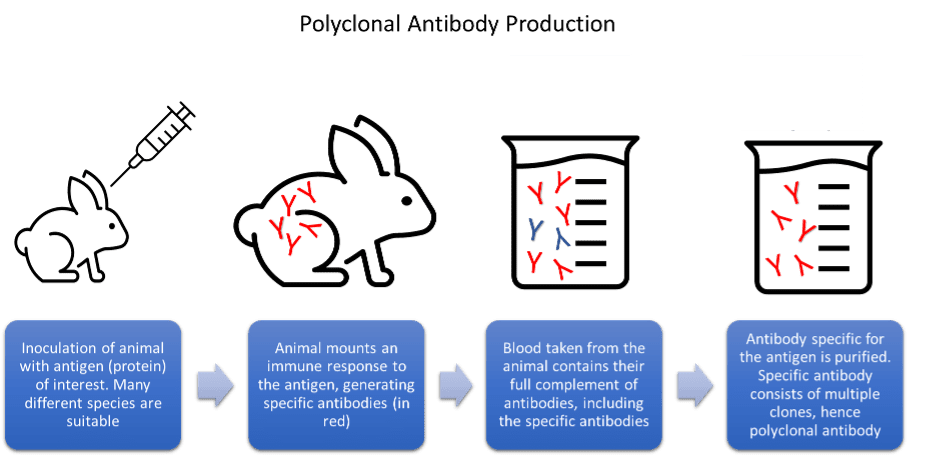
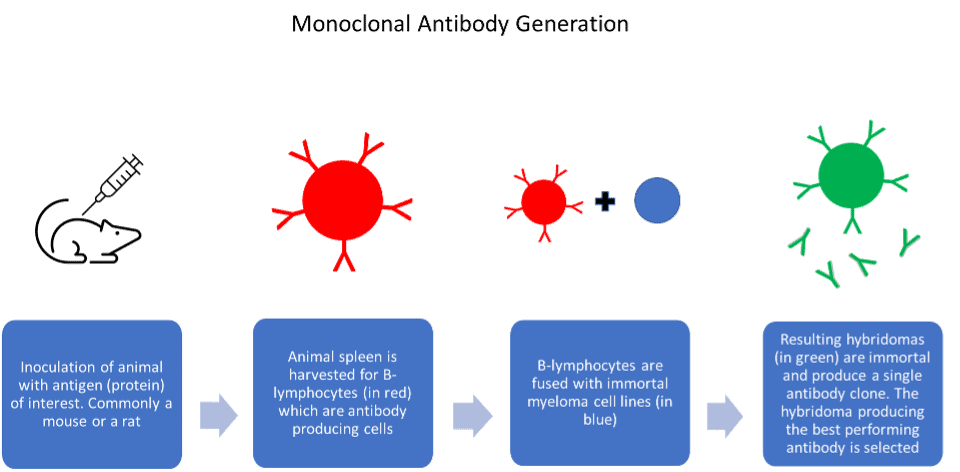
The seminal publication by Köhler and Milstein in 1975, describe the generation of antibody producing hybridomas. This publication has led to what is now a billion-pound industry with antibodies developed as both key research tools and therapeutic agents. However, the very first antibodies used for research predate the invention of monoclonal antibody-producing hybridomas. Prior to this, the mixture of antibodies produced naturally as part of an immune response, were collected directly from the serum of animals and humans. These polyclonal antibodies, and their monoclonal counterparts, were the only source of commercially produced research antibodies before the discovery of recombinant antibodies in the 1990s. This is when a technique called phage display made it possible for antibody manufacturers to easily harness DNA cloning technology to produce antibodies.
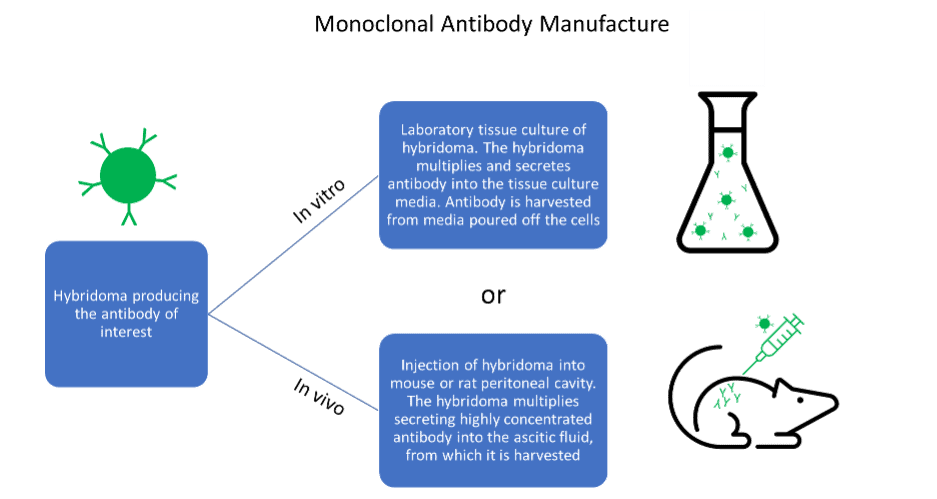
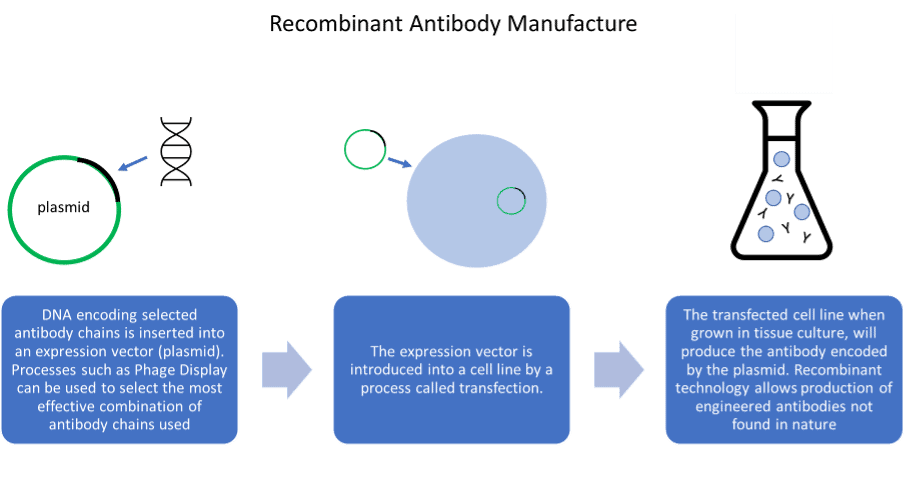
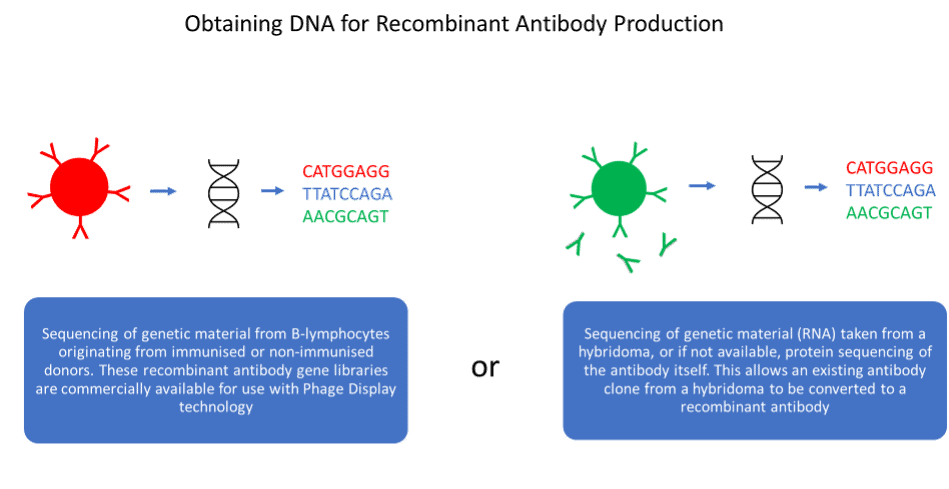
Advantages and disadvantages of antibody generation methods
Each method of antibody generation and manufacture has its advantages and disadvantages. Polyclonal antibodies are high affinity and simple to produce. However, due to the nature of their production, they are only available in finite amounts and eventually run out. Hybridoma cells, which produce monoclonal antibodies in culture, can drift genetically. This can lead to problems reproducing key experiments and give misleading assay results. The hybridoma cells can also die or deteriorate in storage, leading to the permanent loss of key antibody clones. Issues with batch-to-batch variability are also well reported within the scientific community.
Despite this, monoclonal antibodies produced by the in vitro method from hybridoma are an immensely useful tool. They are ubiquitous in research and quick and easy to manufacture in large amounts. On the other hand, antibody manufacture from hybridoma by the in vivo method produces high yields of antibody contained within the ascitic fluid. They are a very reliable method for difficult to produce antibodies. However, the use of ascites as a production method is declining as many regions in Europe have discouraged its use due to animal welfare concerns.
Recombinant antibodies are less well established in the research reagent marketplace but have several advantages over their predecessors. Valuable antibodies cannot be lost as the DNA sequence is known, and antibody engineering becomes possible, allowing antibodies to be designed for specific applications. However, recombinant antibody generation is not necessarily animal-free. Many recombinant antibodies are generated by inoculation of animals, enabling the cloning of its B-cell repertoire to produce antibody gene library. Non-animal derived recombinant antibodies can be created by cloning of the antibody gene repertoire from non-immunised human donors, or libraries can be derived synthetically. Additionally, sequencing technology can be utilised to reproduce antibodies produced by hybridoma, converting key monoclonal antibodies to recombinant antibodies.
References and further reading
1. Köhler G. and Milstein C. (1975) Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256, 495–497
2. Barroso J., Halder M., Whelan M. EURL ECVAM Recommendation on Non-Animal-Derived Antibodies. EUR 30185 EN, Publications Office of the European Union, Luxembourg, 2020, ISBN 978-92-76-18346-4, doi:10.2760/80554, JRC120199.
3. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes Text with EEA relevance [Internet]. 276, 32010L0063 Oct 20, 2010. Available from: http://data.europa.eu/eli/dir/2010/63/oj/eng
4. Fahmy M. A. (2021) Recommendations for the Use of Non-Animal Derived Antibodies instead of Animal-Derived Antibodies: The Effect on the Antibody Research Industry. Master’s Thesis BIMM70. Lund University Faculty of Medicine.